We do not diagnose disease or recommend a treatment protocol or dietary supplement for the treatment of disease. You should share this information with your physician who can determine what nutrition and disease treatment regimen is best for you. Ask your physician any questions you have concerning your medical condition.
You can search this site or the web for topics of interest that I may have written (use Dr Simone and topic).
“We provide truthful information without emotion or influence from the medical establishment, pharmaceutical industry, national organizations, special interest groups or government agencies.” Charles B Simone, M.MS., M.D.
https://tinyurl.com/48u6hvbx
WEIGHT LOSS: GLP-1 PRESCRIPTION DRUGS OR NUTRIENTS LIFESTYLE

Lawrenceville, NJ Dr Simone – GLP-1 agonists are prescription medications and nutrients used for managing type 2 diabetes and aiding in weight loss. Here’s a simplified overview of their effectiveness and sustainability:
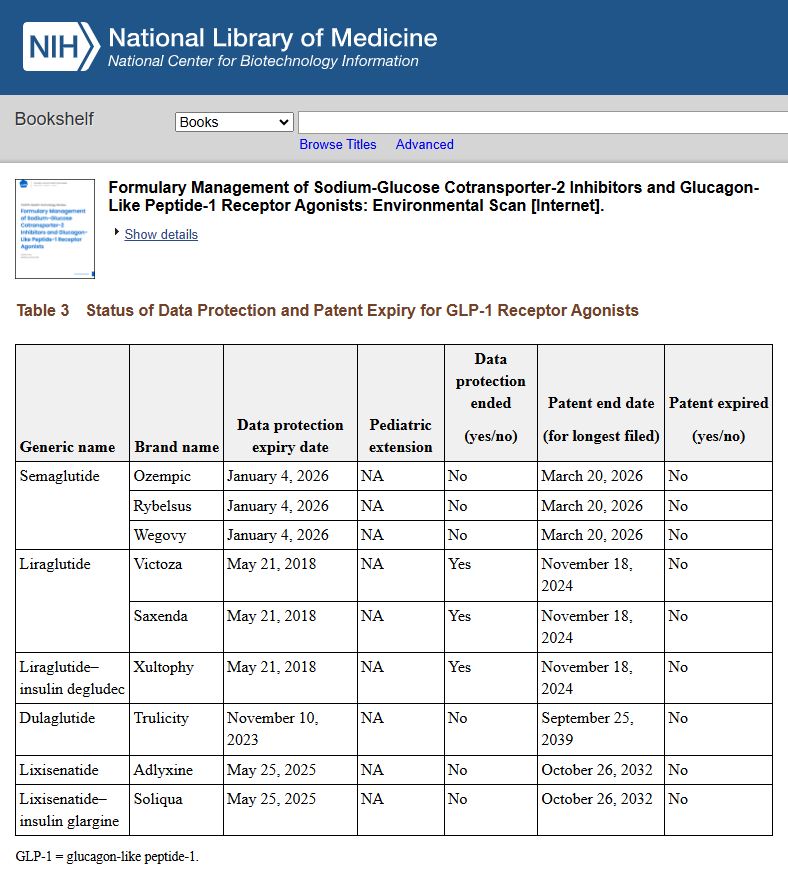
Time is Running Out for Big Pharma’s Patents So They Have Orchestrated a Massive Public Relations Campaign in the United States – they recently obtained weight loss approvals for these drugs in Canada, UK, and India.
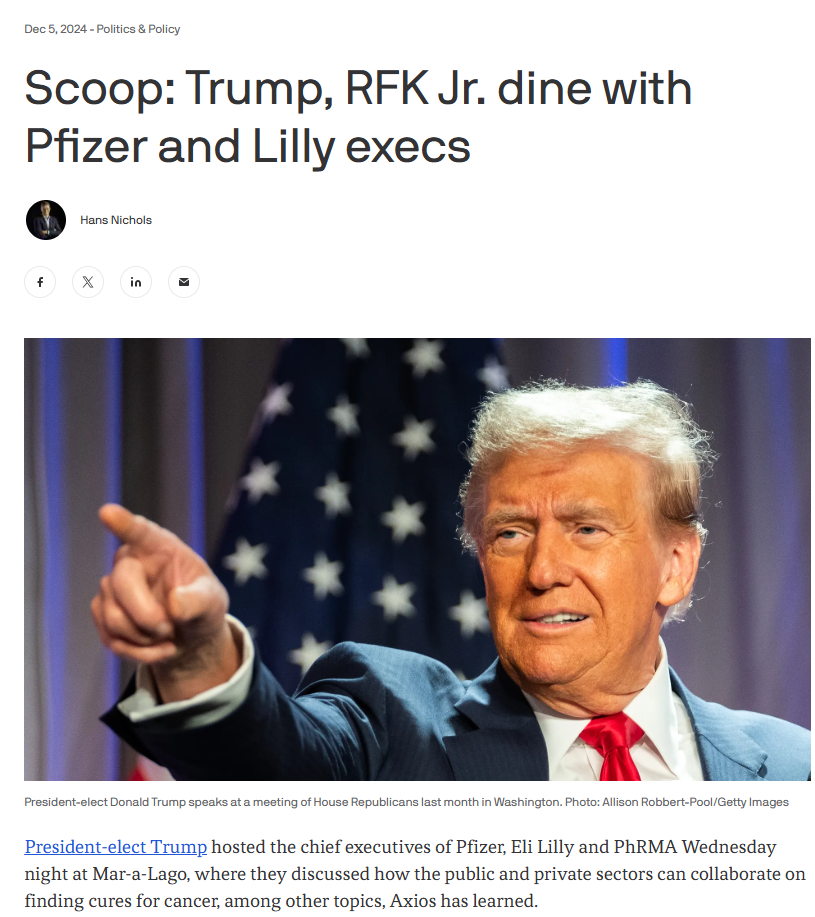
December 4, 2024 – The following attended the dinner at Mar-a-Lago: RFK Jr, Dr Oz (nominee for Centers for Medicare & Medicaid Services), Pfizer CEO Albert Bourla, Lilly CEO David Ricks, Steve Ubl, CEO of the Pharmaceutical Research and Manufacturers of America (PhRMA), and Trump’s incoming chief of staff, Susie Wiles.
Before the election RFK Jr was critical of these drugs, now he says lifestyle changes are most important but GLP-1 drugs “have a place.”
On or about Dec 10 @elonmusk posted on X: “Nothing would do more to improve the health, lifespan, and quality of life for Americans than making GLP inhibitors super low cost to the public. Nothing else is even close.”.
December 14, 2924 – SOUNDS LIKE A COMMERCIAL TO ME DR SEIGEL AND FOX NEWS FOR BIG PHARMA FOR THESE GLP-1 DRUGS and naming @elonmusk as an advocate and Medicare https://x.com/i/status/1868077658401304866 https://www.foxnews.com/video/6366010257112
https://www.foxnews.com/video/6366010257112
February 9, 2025 – But then President Trump says this:
https://rumble.com/embed/v6klul6/?pub=hs2n7
“It’s very unfair, the identical package made in the same factory shipped to different places, but made literally in the same factory. In London it’s [GLP-1 drugs] $88 and in New York it’s $1,200 and you can’t get it. And the reason is because everything is added on to the United States because the United States has been too nice… I’m going to solve that problem one way or the other…it’s not fair in other countries, you go to Canada all of the drugs are much less expensive. I suggested that some of the governors should go buy their drugs in Canada and send them back to here.”
NOW LET’S LOOK AT THE FACTS
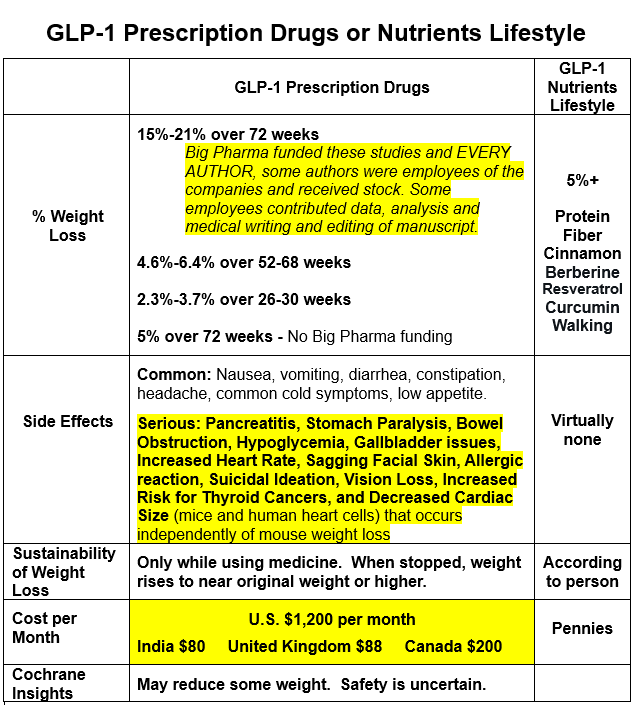
Maximum Weight Loss Recorded for Prescription Drugs:
Semaglutide (Wegovy): Users have lost around 15-16% of their body weight over 68-76 weeks, with some maintaining this loss for 6 months post-treatment. However, much of this weight is regained within a year of stopping the drug. The study was funded by Novo Nordisk and EVERY AUTHOR received money from Novo Nordisk and other companies, some authors were employees of Novo Nordisk and received stock.
Liraglutide (Saxenda): Average weight reduction is about 6.4% over 68 weeks, less than semaglutide in direct comparisons. The study was funded by Novo Nordisk and EVERY AUTHOR received money from Novo Nordisk and other companies, some authors were employees of Novo Nordisk and received stock.
Tirzepatide (Mounjaro/Zepbound): Can lead to an average weight loss of 18-21% over 72-78 weeks, being particularly effective for diabetic patients. Eli Lilly funded the study and EVERY AUTHOR, all but two were employees of Eli Lilly and some received stock. And “We thank Weiguo Zhu and Xiaonan Guo (Eli Lilly) for their contributions to the data preparation and analysis and Chrisanthi A. Karanikas (Eli Lilly) for medical writing and editing assistance with an earlier draft of the manuscript.”
Dulaglutide (Trulicity): Patients with type 2 diabetes lost an average of 4.6% of their body weight over 52 weeks on a 3 mg dose.
Exenatide (Byetta, Bydureon): Exenatide has shown modest weight loss, with studies reporting an average of 2.3% to 3.7% weight loss over 26 to 30 weeks.
Sustainability of Weight Loss:
Long-term Use: Weight loss is sustainable while the medication is continued. Stopping the drug often leads to significant weight regain, sometimes up to two-thirds of the lost weight or more within a year.
Maintenance Phase: Weight loss typically plateaus after the first year, but lower caloric intake might persist, suggesting some behavioral changes in eating habits.
Tapering Off: Gradual reduction can help maintain some weight loss longer than immediate cessation.
Real-World vs. Clinical Trials:
In clinical settings, weight loss is more pronounced due to controlled conditions. Real-world results show around 5% weight loss at 72 weeks, influenced by adherence and lifestyle. No funding from Big Pharma.
Side Effects and Complications:
Common: Nausea, vomiting, diarrhea, constipation, headache, common cold symptoms, low appetite.
Serious:
-
-
-
Pancreatitis
-
Stomach Paralysis
-
Bowel Obstruction
-
Hypoglycemia
-
Gallbladder issues
-
Increased Heart Rate
-
Sagging Facial Skin
-
Allergic reaction
-
Suicidal Ideation
-
Vision Loss(people taking Ozempic were more than twice as likely to have vision loss as people who took another diabetic drug https://www.medrxiv.org/content/10.1101/2024.12.09.24318574v1.full.pdf),
-
Increased Risk for Thyroid Cancers (Glucagon-like peptide 1 receptor agonists and thyroid cancer: is it the time to be concerned? https://pmc.ncbi.nlm.nih.gov/articles/PMC10563602/)
-
These drugs are contraindicated in patients with a personal or family history of medullary thyroid carcinoma or multiple endocrine neoplasia syndrome type 2.
-
-
-
-
-
-
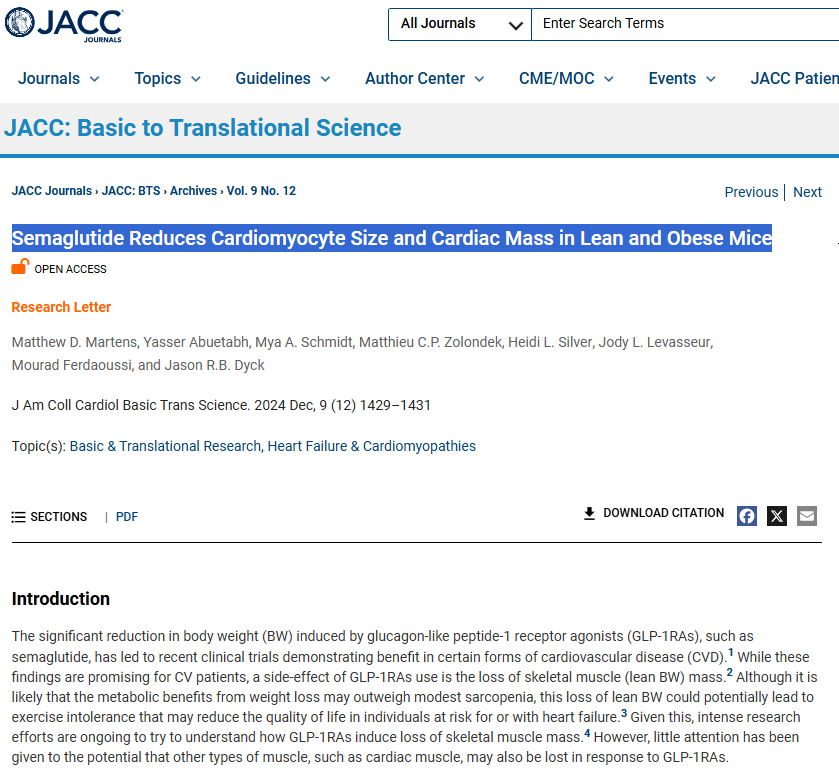
Decreased Cardiac Size (mice and human heart cells) that occurs independently of mouse weight loss (https://www.jacc.org/doi/10.1016/j.jacbts.2024.07.006).
-
-
Funding and Research:
Pharmaceutical companies like Novo Nordisk and Eli Lilly fund many studies and authors of those studies, raising concerns about potential bias towards highlighting benefits and focusing less on side effects or limitations.
For instance, a study cited showed that semaglutide resulted in significant weight loss compared to liraglutide, and this study was funded by Novo Nordisk, the maker of semaglutide. Similarly, research on tirzepatide, another GLP-1 agonist, has been supported by Eli Lilly, its manufacturer. There are also studies conducted by academic institutions or funded by independent grants where the influence of pharmaceutical companies might be less direct. However, even in these cases, researchers often collaborate with or receive support from industry in some capacity. Therefore, transparency is key in evaluating study outcomes.
Cochrane Library Insights:
Effectiveness: GLP-1 agonists “probably reduce glucose levels and may reduce weight. The safety of GLP-1 agonists is uncertain.” https://www.cochrane.org/CD011798/RENAL_glucose-lowering-medications-treat-diabetes-and-chronic-kidney-disease
Long-term Complications: Evidence on long-term complications like pancreatitis or thyroid cancer is not conclusive, with ongoing discussions about gastrointestinal side effects and cardiovascular benefits.
In summary, GLP-1 agonist prescription medicines can lead to weight loss, but maintaining this loss largely depends on continued medication use. They come with various side effects, some of which can be serious in the long term. The influence of industry funding on research outcomes is a noted concern.
NUTRIENTS can stimulate the release of GLP-1
PROTEIN, FAT, and FIBER: These key nutrients slow digestion and increase GLP-1 levels. Foods rich in these include eggs, high-fiber grains like oats and barley, nuts, and avocados. https://www.webmd.com/obesity/features/natural-glp1-boosters
Weight Reduction Evidence:
Protein: High protein intake is associated with increased satiety, reduced hunger, and consequently, may lead to weight loss by reducing caloric intake. Whey protein, in particular, has been shown to increase GLP-1 and PYY, hormones associated with satiety
Fat: Moderate intake of healthy fats (like those from the Mediterranean diet) has been linked with better weight management outcomes compared to low-fat diets, suggesting a complex relationship where fat type and quality matter.
Fiber: Dietary fiber, especially from sources like psyllium or guar gum, can aid in weight loss by promoting satiety, reducing caloric intake, and possibly altering gut microbiota to favor weight loss.
A Systematic Review of Dietary Supplements and Alternative Therapies for Weight Loss https://pmc.ncbi.nlm.nih.gov/articles/PMC8231729/
CINNAMON (preferably from Ceylon [Sri Lanka] https://www.simonesuperenergy.com/cinnamon-improves-glucose-and-lipid-levels/): Consuming 3 grams with a meal can increase GLP-1 while reducing insulin levels without affecting blood sugar significantly.
Weight Reduction Evidence: Several studies suggest that cinnamon can have a beneficial effect on weight management. A systematic review and meta-analysis of randomized controlled trials (RCTs) demonstrated that cinnamon supplementation can significantly decrease body weight, BMI, waist circumference, and fat mass. One study showed a significant reduction in body weight by -1.02 kg, BMI by -0.51 kg/m², waist circumference by -2.40 cm, and fat mass by -1.02% compared to placebo groups.
Cinnamon: Potential Role in the Prevention of Insulin Resistance, Metabolic Syndrome, and Type 2 Diabetes. https://pmc.ncbi.nlm.nih.gov/articles/PMC2901047/#:~:text=The%20ingestion%20of%203%20grams,emptying%20rate%20in%20healthy%20subjects.
Cinnamon supplementation positively affects obesity: A systematic review and dose-response meta-analysis of randomized controlled trials https://www.sciencedirect.com/science/article/abs/pii/S0261561419300718.
BERBERINE: Helps manage blood sugar and insulin resistance, an optimal dose of 1 gram daily can support weight loss, while higher doses can further benefit lipid profiles and insulin sensitivity.
Weight Reduction Evidence: Berberine has been shown in multiple studies to aid in weight loss. A review of 12 studies found that berberine supplementation led to significant reductions in body weight, BMI, and belly fat. It may work by activating AMP-activated protein kinase (AMPK), which can promote weight loss by enhancing fat oxidation and inhibiting fat storage. Another study specifically highlighted berberine’s influence on gut microbiota, which may contribute to weight loss.
Effects of berberine on glucose-lipid metabolism, inflammatory factors and insulin resistance in patients with metabolic syndrome. https://pmc.ncbi.nlm.nih.gov/articles/PMC6434235/#:~:text=Berberine%20improves%20physiological%20stimulation%20of,fat%20to%20insulin%20(16).
The effect of Berberine on weight loss in order to prevent obesity: A systematic review https://www.sciencedirect.com/science/article/pii/S0753332220303292
Berberine – A Powerful Supplement With Many Benefits https://www.healthline.com/nutrition/berberine-powerful-supplement;
RESVERATROL: Can significantly boost GLP-1 levels in diabetic subjects, bringing levels close to those in healthy controls. Dose varies between 1,000 mg to 2,000 mg per day.
Weight Reduction Evidence: Resveratrol has demonstrated potential in weight management, particularly in animal studies. It increases SIRT-1 expression, which can reduce adipogenesis and modulate lipid metabolism in adipocytes. In human studies, resveratrol supplementation has been linked to decreased fat mass, though results are not universally consistent.
Current evidence to Propose Different Food Supplements for Weight Loss: A Comprehensive Review https://pmc.ncbi.nlm.nih.gov/articles/PMC7551574/
Effects of Resveratrol on Metabolic Indicators in Patients with Type 2 Diabetes: A Systematic Review and Meta-Analysis https://pmc.ncbi.nlm.nih.gov/articles/PMC9158797/
Pilot Study of Resveratrol in Older Adults With Impaired Glucose Tolerance https://pmc.ncbi.nlm.nih.gov/articles/PMC3670158/
Probiotic and resveratrol normalize GLP-1 levels and oxidative stress in the intestine of diabetic rats. https://pmc.ncbi.nlm.nih.gov/articles/PMC8091914/#:~:text=Our%20results%20also%20showed%20that,compared%20to%20the%20diabetic%20group.
Research Helps Unlock the Secrets of Weight Management https://www.iherb.com/blog/research-helps-unlock-the-secrets-of-obesity/151
PSYLLIUM: A rich source of soluble fiber, which indirectly increases GLP-1.
CURCUMIN: Found in turmeric, it can boost GLP-1 secretion.
Weight Reduction Evidence: Curcumin from turmeric has been studied for its weight loss properties. It can induce adiponectin secretion, inhibit adipocyte differentiation, and possess insulin-sensitizing effects. A double-blind, randomized, placebo-controlled trial reported significant weight loss and reduction in body fat percentage with curcumin supplementation.
Current Evidence to Propose Different Food Supplements for Weight Loss: A Comprehensive Review https://pmc.ncbi.nlm.nih.gov/articles/PMC7551574/
EXERCISE: Regular physical activity, regardless of intensity, appears to raise GLP-1 levels. https://pmc.ncbi.nlm.nih.gov/articles/PMC6107470/#:~:text=Although%20the%20effect%20of%20light,levels%20irrespective%20of%20its%20intensity.
Conclusion:
GLP-1 agonists are prescription medications and nutrients used for managing type 2 diabetes and aiding in weight loss. Natural compounds like protein and fiber, and cinnamon, berberine, resveratrol, curcumin together with regular walking contribute to weight loss by naturally enhancing GLP-1 levels, supporting metabolic health and aiding in diabetes and weight management in the absence of significant adverse events and are accessible without prescription. The prescription medications can lead to weight reductions but Big Pharma funded the studies and EVERY AUTHOR, some of whom were employees, and some employees contributed to data preparation and analysis and medical writing and editing assistance of the manuscript. Also, these medications come with a higher cost and side effects.
REFERENCES For GLP-1 Agonist Prescription Drugs
Semaglutide (Wegovy):
Wilding, J. P., et al. (2021). “Once-Weekly Semaglutide in Adults with Overweight or Obesity.” New England Journal of Medicine, 384(11), 989-1002.
Novo Nordisk funded the study and EVERY AUTHOR, some authors were employees of Novo Nordisk and received stock: Wilding JPH, Batterham RL, Calanna S, Davies M, Van Gaal LF, Lingvay I, McGowan BM, Rosenstock J, Tran MTD, Wadden TA, Wharton S, Yokote K, Zeuthen N, Kushner RF https://www.nejm.org/doi/suppl/10.1056/NEJMoa2032183/suppl_file/nejmoa2032183_disclosures.pdf
Rubino, D., et al. (2022). “Effect of Continued Weekly Subcutaneous Semaglutide vs Placebo on Weight Loss Maintenance in Adults With Overweight or Obesity: The STEP 4 Randomized Clinical Trial.” JAMA, 327(14), 1414-1425.
ClinicalTrials.gov ID CT03548987 Sponsor Novo Nordisk A/S Information provided by Novo Nordisk A/S (Responsible Party) 2022-01-19
Conflict of Interest Disclosures: Dr Rubino reported being a clinical investigator for Boehringer Ingelheim and AstraZeneca and receiving speaker fees, consulting fees, and honoraria from Novo Nordisk and being a shareholder in Novo Nordisk. Dr Davies reported receipt of consultant, advisory board member, and speaker fees from Novo Nordisk, Sanofi, Eli Lilly, and Boehringer Ingelheim; advisory board member and speaker fees from AstraZeneca; advisory board member fees from Gilead Sciences, Janssen, and Lexicon; speaker fees from Napp Pharmaceuticals and Takeda Pharmaceuticals International, and grants from AstraZeneca, Novo Nordisk, Boehringer Ingelheim, Janssen, and Sanofi. Dr Hesse reported receipt of personal fees from Novo Nordisk. Dr Greenway reported receipt of grants from Pennington Biomedical Research Center and NuSirt to his institution; research funding from NovMeta Pharma and Melior Discoveries; receipt of consulting fees from Basic Research, Dr. Reddy’s Laboratories, Jazz Pharmaceuticals, General Nutrition Corporation, and Regeneron Pharmaceuticals; receipt of scientific advisory board fees from Jenny Craig and Pfizer; and stock ownership in Academic Technology Ventures, Ketogenic Health Systems, Plensat, UR Labs, and Rejuvenate Bio. In addition, Dr Greenway has a patent issued for orlistat and a patent pending for pramlintide/albuterol. Ms Jensen reported receipt of personal fees from Novo Nordisk. Dr Lingvay reported receipt of grants, personal fees, and nonfinancial support from Novo Nordisk and Sanofi; personal fees and nonfinancial support from Eli Lilly, AstraZeneca, and Boehringer Ingelheim; personal fees from Janssen, Intercept, Intarcia, TARGETPharma, Mannkind, Valeritas, Bayer, and Zealand Pharma; grants and nonfinancial support from Merck and Pfizer; and grants from Mylan. Dr Mosenzon reported receipt of grants from Novo Nordisk and AstraZeneca through Hadassah Medical Center; advisory board and speaker’s bureau fees from Novo Nordisk, AstraZeneca, Eli Lilly, Merck Sharp & Dohme, and Sanofi; speaker’s bureau fees from Boehringer Ingelheim and Janssen; and advisory board fees from BOL Pharma. Dr Rosenstock reported receipt of scientific advisory board fees, honoraria, consulting fees, and grants/research support from Novo Nordisk, Applied Therapeutics, Boehringer Ingelheim, Eli Lilly, Intarcia, Oramed, and Sanofi; honoraria or consulting fees from Zealand; and grants/research support from Genentech, Novartis, Pfizer, REMD Biotherapeutics, and vTv Therapeutics. Dr Rubio reported receipt of personal fees from Novo Nordisk. Dr Tadayon reported being a full-time employee of and shareholder in Novo Nordisk. Dr Wadden reported receipt of grants from Novo Nordisk received on behalf of the University of Pennsylvania and scientific advisory board fees from Novo Nordisk and WW (formerly Weight Watchers). Dr Dicker reported receipt of personal fees, nonfinancial support, and grants from Novo Nordisk and grants from Eli Lilly. No other disclosures were reported.
Liraglutide (Saxenda):
Pi-Sunyer, X., et al. (2015). “A Randomized, Controlled Trial of 3.0 mg of Liraglutide in Weight Management.” New England Journal of Medicine, 373(1), 11-22.
Study Funded by Novo Nordisk; Authors: Xavier Pi-Sunyer, M.D., Arne Astrup, M.D., D.M.Sc., Ken Fujioka, M.D., Frank Greenway, M.D., Alfredo Halpern, M.D., Michel Krempf, M.D., Ph.D., David C.W. Lau, M.D., Ph.D., Carel W. le Roux, F.R.C.P., Ph.D., Rafael Violante Ortiz, M.D., Christine Bjørn Jensen, M.D., Ph.D., and John P.H. Wilding, D.M.
Supported by Novo Nordisk: Dr. Pi-Sunyer reports receiving fees for serving on advisory boards from Novo Nordisk, Eli Lilly, Weight Watchers, and Johnson & Johnson. Dr. Astrup reports receiving fees for serving on advisory boards from Boehringer Ingelheim and Pathway Genomics, fees for serving on a study committee from Vivus, and consulting fees from Novo Nordisk, Arena Pharmaceuticals, Basic Research, Gelesis, Orexigen Therapeutics, Pfizer, and S-Biotek. Dr. Fujioka reports receiving fees for serving on an advisory board from Novo Nordisk, consulting fees from Eisai, NPS Pharmaceuticals, Vivus, Isis, NaZura, Novo Nordisk, Zafgen, and Orexigen Therapeutics, lecture fees from Eisai, NPS Pharmaceuticals, Vivus, Abbott, and Takeda, and grant support from Eisai, NPS Pharmaceuticals, Novo Nordisk, Orexigen Therapeutics, EnteroMedics, Shire, and Weight Watchers. Dr. Greenway reports receiving fees for serving on advisory boards from Jenny Craig/Curves, Novo Nordisk, Orexigen Therapeutics/Takeda, Pemlab, and Zafgen, fees for serving on a data safety monitoring board from Baranova, fees for serving on the editorial board of Diabetic Living, consulting fees from Baranova, Basic Research, Eisai, General Nutrition Corporation, Japan Tobacco, Novo Nordisk, Obalon Therapeutics, and Orexigen Therapeutics/Takeda, lecture fees from the American Society of Bariatric Physicians and Vindico Medical Education, and holding stock/stock options in Neothetics, Microbiome Therapeutics, and PlenSat. In addition, he reports patents related to potato chips without fat (U.S. Patent No. 5,952,026), a pyruvate delivery system (U.S. Patent No. 6,417,231), a waist chain (U.S. Patent No. 7,150,141), gallic acid for angiogenesis inhibition (U.S. Patent No. 7,709,031, serial no. 10/559,091), and pomegranate angiogenesis inhibition (U.S. Patent No. 8,334,000 B2), and pending patents related to methionine-restricted food (Application No. PTC/US2014/040790) and caffeine and albuterol synergy (Application No. U.S.P.T.O.61/896,922). Dr. Halpern reports receiving lecture fees from Novo Nordisk. Dr. Jensen is an employee of and holds stock in Novo Nordisk. Dr. Lau reports receiving fees for serving on advisory boards from AstraZeneca, Boehringer Ingelheim, Bristol-Myers Squibb, Eli Lilly, Novo Nordisk, Janssen, Roche, Valeant, and Amgen, lecture fees from AstraZeneca, Boehringer Ingelheim, Bristol-Myers Squibb, Eli Lilly, Novo Nordisk, Janssen, Valeant, Amgen, and Merck, and grant support from AstraZeneca, Boehringer Ingelheim, BristolMyers Squibb, Eli Lilly, and Novo Nordisk. Dr. Violante Ortiz reports receiving fees for serving on advisory boards from Merck Sharp & Dohme, Boehringer Ingelheim, Novo Nordisk, BristolMyers Squibb, AstraZeneca, and Eli Lilly, lecture fees from Merck Sharp & Dohme, Boehringer Ingelheim, Novo Nordisk, Bristol Myers Squibb, and AstraZeneca, and grant support from Novo Nordisk and Eli Lilly. Dr. Wilding reports receiving fees for serving on advisory boards from Novo Nordisk, Astellas, BristolMyers Squibb, and Janssen, consulting fees through his institution from Pfizer, lecture fees from Novo Nordisk, Boehringer Ingelheim, Bristol-Myers Squibb, Janssen, Eli Lilly, and Merck, and grant support from Bristol-Myers Squibb. No other potential conflict of interest relevant to this article was reported.
Comparison with Semaglutide: Wilding, J. P., et al. (2021). “Once-Weekly Semaglutide in Adults with Overweight or Obesity.” New England Journal of Medicine, 384(11), 989-1002.
Tirzepatide (Mounjaro/Zepbound):
Frías, J. P., et al. (2023). “Tirzepatide versus Semaglutide Once Weekly in Patients with Type 2 Diabetes: A Randomized, Open-Label, Phase 3 Trial.” The Lancet, 401(10391), 2057-2068.
The study was Funded by Eli Lilly; EVERY AUTHOR of the study received money from Eli Lilly and other companies, all but two were actual employees of Eli Lilly and some received stock: Authors: Juan P. Frías, M.D., Melanie J. Davies, M.D., Julio Rosenstock, M.D., Federico C. Pérez Manghi, M.D., Laura Fernández Landó, M.D., Brandon K. Bergman, Pharm.D., Bing Liu, Ph.D., Xuewei Cui, Ph.D., and Katelyn Brown, Pharm.D. Author Disclosure forms provided https://www.nejm.org/doi/suppl/10.1056/NEJMoa2107519/suppl_file/nejmoa2107519_disclosures.pdf
“We thank Weiguo Zhu and Xiaonan Guo (Eli Lilly) for their contributions to the data preparation and analysis and Chrisanthi A. Karanikas (Eli Lilly) for medical writing and editing assistance with an earlier draft of the manuscript.”
Rosenstock, J., et al. (2021). “Efficacy and safety of once-weekly tirzepatide versus insulin glargine by baseline HbA1c (SURPASS-3): a subgroup analysis from a randomized controlled trial in type 2 diabetes.” Diabetes Care, 44(11), 2489-2496.
Dulaglutide (Trulicity): Ludvik, B., et al. (2021). “Dulaglutide as add-on therapy to SGLT2 inhibitors in patients with inadequately controlled type 2 diabetes (AWARD-11): a 24-week, randomized, double-blind, placebo-controlled trial.” Diabetes, Obesity and Metabolism, 23(1), 27-36.
Exenatide (Byetta, Bydureon):
Blevins, T., et al. (2011). “A Phase III Study of Exenatide Once Weekly Administered Alone or in Combination With Metformin or Pioglitazone in Patients With Type 2 Diabetes.” Diabetes Care, 34(10), 2144-2150.
Drucker, D. J., et al. (2008). “Exenatide once weekly versus twice daily for the treatment of type 2 diabetes: a randomised, open-label, non-inferiority study.” The Lancet, 372(9645), 1240-1250.
Real-World Data on Weight Loss Sustainability:
White, G. E., et al. (2023). “Real-world weight loss effectiveness of GLP-1 agonists among patients with type 2 diabetes: a retrospective cohort study.” PMC.
Research reported in this publication was supported by the National Center For Advancing Translational Sciences of the National Institutes of Health under Award Number KL2TR001856 and the National Institute of Diabetes and Digestive and Kidney Diseases (Luo) under Award Number K23DK120956. Dr. White, Ms. Shu, Dr. Arnold, and Dr. Korytkowski have no conflicts to disclose. Dr. Luo received consulting fees from Health Action International and Alosa Health outside the scope of this work. Dr. Rometo received consulting fees from Nestle Health Sciences outside the scope of this work.
Other References
Vision Loss: Simonsen E, et al. 2024. Use of semaglutide and risk of non-arteritic anterior ischemic optic neuropathy: A Danish–Norwegian cohort study. https://www.medrxiv.org/content/10.1101/2024.12.09.24318574v1.full.pdf

Increased Risk for Thyroid Cancers (Glucagon-like peptide 1 receptor agonists and thyroid cancer: is it the time to be concerned? https://pmc.ncbi.nlm.nih.gov/articles/PMC10563602/)
Decreased Cardiac Size (mice and human heart cells) that occurs independently of mouse weight loss (https://www.jacc.org/doi/10.1016/j.jacbts.2024.07.006). “Moreover, cultured human cardiomyocytes (AC16 cells) treated with semaglutide (10 nmol/L, 24 hours) showed reduced cardiomyocyte area (Figure 1A, xi), demonstrating a direct cardiomyocyte autonomous effect of semaglutide, that is independent of potential changes in workload that may have occurred in vivo.”